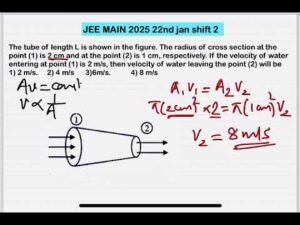
Description
****2025 complete solution playlist**😃🤘🏻
***All JEE Main/AIEEE questions playlist***😃🤘🏻
***All NEET PYQs playlist***😃🤘🏻
✅✅✅😃You can also find for chapterwise playlist of JEE MAIN and NEET on the channel👍🏻👍🏻✅✅✅
#jee,#jeemain,#iitjee,#neet,#pyq,#importantquestions,#jeemainpapersolution,#jeemain2025,#physicsmonk
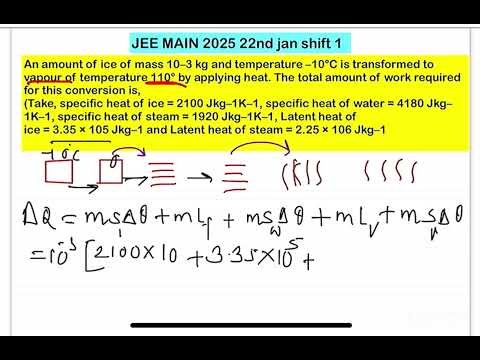
An amount of ice of mass 10–3 kg and temperature –10°C is transformed to vapour of temperature 110° by applying heat. The total amount of work required for this conversion is,
(Take, specific heat of ice = 2100 Jkg–1K–1, specific heat of water = 4180 Jkg–1K–1, specific heat of steam = 1920 Jkg–1K–1, Latent heat of
ice = 3.35 × 105 Jkg–1 and Latent heat of steam = 2.25 × 106 Jkg–1
Welcome to PhysicsMonk.com – India’s Largest Platform for Instant Doubt Solutions
PhysicsMonk.com is the ultimate destination for instant video solutions to your doubts in Physics—from Class 11th to JEE Advanced and NEET level. With a growing library of over 15 lakh+ expert-created video solutions, we make learning fast, easy, and effective.
Just click a photo of your doubt using the PhysicsMonk in the end and submit in google, and get an instant, step-by-step video explanation. It’s the simplest way to solve doubts in seconds—whether you’re studying for CBSE board exams, IIT-JEE, NEET, or any other competitive exam.
Why PhysicsMonk.com?
• 10,00,000+ Free Video Solutions
• Covers NCERT, JEE Main, JEE Advanced, NEET, and State Boards
• Unlimited Doubt Solving with Just One Click
• Instant, Concept-Based Answers by Experts
• 24×7 Learning Support via the AskDoubtnut App
Trusted by millions of students across India, PhysicsMonk.com helps you turn doubts into confidence. No more waiting—ask, learn, and grow with PhysicsMonk.com!
#DoubthotoDoubtnutkaro