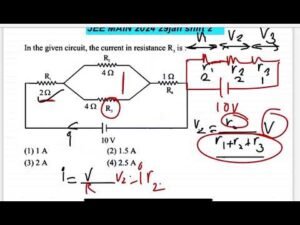
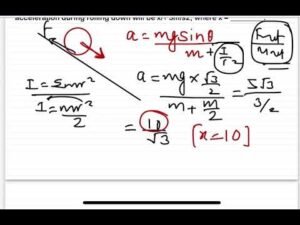
Description
*29th jan shift1 JEE MAIN Solutions****
****2024 complete solution playlist**😃🤘🏻
*****2025 complete solution playsit*****
***All JEE Main/AIEEE questions playlist***😃🤘🏻
***All NEET PYQs playlist***😃🤘🏻
✅✅✅😃You can also find for chapterwise playlist of JEE MAIN and NEET on the channel👍🏻👍🏻✅✅✅
#jee,#jeemain,#iitjee,#neet,#pyq,#importantquestions,#jeemainpapersolution,#jeemain2025,#physicsmonk
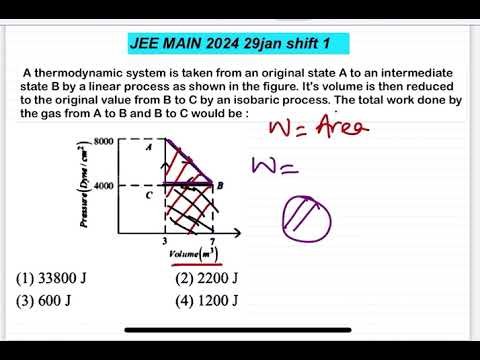
A thermodynamic system is taken from an original state A to an intermediate state B by a linear process as shown in the figure. It’s volume is then reduced to the original value from B to C by an isobaric process. The total work done by the gas from A to B and B to C would be :
#DoubthotoDoubtnutkaro