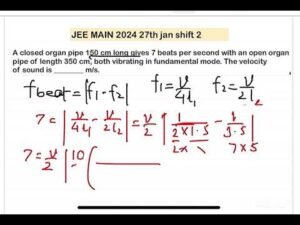
Description
*2024 (27th jan shift2) playslist*****👍🏻

****2024 complete solution playlist**😃🤘🏻

*****2025 complete solution playsit*****

***All JEE Main/AIEEE questions playlist***😃🤘🏻
You might also like:
Description *2024 (27th jan shift2) playslist*****👍🏻 https://youtube.com/playlist?list=PLJlL0nazku91YhYAHPWliNUfw2TjP8PM4&si=bYACuvs1ZX5M0dkm...
Read More Description *2024 (27th jan shift1) playslist*****👍🏻 https://youtube.com/playlist?list=PLJlL0nazku90yc5A5IO6ZLbaMzqejjABq&si=WCh8IR5RJgj8IrDN...
Read More Description *2024 (27th jan shift1) playslist*****👍🏻 https://youtube.com/playlist?list=PLJlL0nazku90yc5A5IO6ZLbaMzqejjABq&si=WCh8IR5RJgj8IrDN...
Read More Description *2024 (27th jan shift2) playslist*****👍🏻 https://youtube.com/playlist?list=PLJlL0nazku91YhYAHPWliNUfw2TjP8PM4&si=bYACuvs1ZX5M0dkm...
Read More Description *2024 (27th jan shift2) playslist*****👍🏻 https://youtube.com/playlist?list=PLJlL0nazku91YhYAHPWliNUfw2TjP8PM4&si=bYACuvs1ZX5M0dkm...
Read More Description *2024 (27th jan shift2) playslist*****👍🏻https://youtube.com/playlist?list=PLJlL0nazku91YhYAHPWliNUfw2TjP8PM4&si=bYACuvs1ZX5M0dkm****2024 complete...
Read More
***All NEET PYQs playlist***😃🤘🏻

✅✅✅😃You can also find for chapterwise playlist of JEE MAIN and NEET on the channel👍🏻👍🏻✅✅✅
#jee,#jeemain,#iitjee,#neet,#pyq,#importantquestions,#jeemainpapersolution,#jeemain2025,#physicsmonk
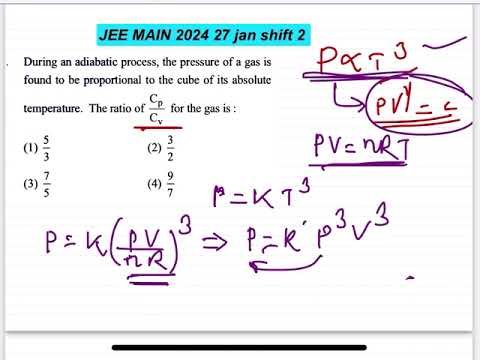
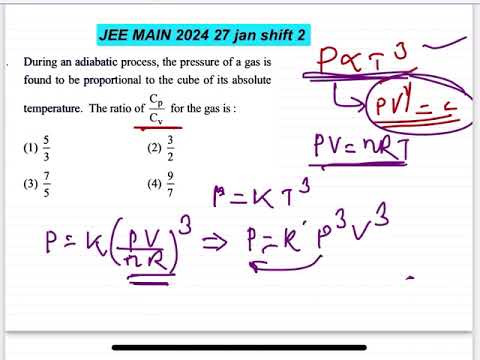
During an adiabatic process, the pressure of a gas is
found to be proportional to the cube of its absolute
temperature. The ratio of Cp and Cv for the gas is